Skyepharma funds a PhD thesis in collaboration with Bordeaux University, with the aim to expand deep physical and mechanical comprehension of complex tablet behaviors, with a specific focus on press coated tablets.
INTRODUCTION
With growing interest in chronotherapeutics, the technologies of modified drug release become of major importance, as well as the good control on their release profile. Particularly, the press-coated tablets are a mean to achieve a delayed release [1], [2], i.e. a delay of several hours after the tablet administration during which no drug is released followed by a fast pulsatile release of the active substance. This delay is often called lag-time and is one of the main quality attributes to be managed to reach the therapeutic goal. Previous works have shown that the lag-time is adjustable by modifying the shell powder’s formulation [1]–[3]. Recent works have also shown that coating parameters like pressure, layer thickness and coat diameter can a have high impact on the shell structure of the press-coated tablets [4]. Thus, such process parameters are likely to influence the lag-time, which will be studied in this work.
Depending on the therapeutic application, it might also be important to ensure a quick release of the drug after the lag-time, e.g. 80% of the drug quantity in 30 minutes. In this work, the dissolution profile of the active agent was followed along with the visual opening mode of the shell during the dissolution test in order to demonstrate the links between process parameters, opening mode and dissolution kinetics.
MATERIALS AND METHODS
Powders and tablet manufacturing
As a commercial formulation was used for the experiments of this study, the composition of the powders may not be precisely detailed. The powder for the core compression contains a dose of a model active ingredient, mixed with non-active excipients. The powder used as the shell of the press-coated tablet is a mix containing no active ingredient, that is non-soluble and hydrophobic once compressed into a compact.
All compressions were performed on a compaction simulator Styl’One Evolution (Medelpharm, Beynost, France), using different euro B punches: chamfered round flat with a diameter of 5mm for the core compression and round flat with a diameter of 10mm for the coating compression. The cores were compacted at a pressure of 360MPa
For the coating compression, the core was placed manually on the center of the barrier powder bed, before the second filling and main compression. The main compression levels were set to get pressures of 25, 50, 75 MPa. The filling heights were adjusted to have a controlled final layer thickness (i.e. the distance between the core surface and the shell surface) in order to study its influence.
Dissolution test
The dissolution experiments were performed with a AT7 dissolution tester (Sotax, Aesch, Switzerland). The press-coated tablets were individually immersed in 1000mL of purified water, heated at 37°C. The rotation rate of the paddle was set to 100rpm.
Analysis
To follow the drug release rate of the press-coated tablet, several samples of 2mL were regularly taken before, during and after the opening of the shell. The drug concentration in the samples was measured with a spectrophotometer GENESYS 10S UV-Vis (Thermo Fischer Scientific, Waltham, USA). The analysis was performedat a wavelength of 242nm which corresponds to the maximum absorbance of the model drug. The dissolution of a placebo core was previously made to make sure that no other excipient of the core absorbs at the selected wavelength.
Conjointly to the spectrophotometry, the dissolutions were visually followed using a camera (Lumix DC-G9, Panasonic, Kadoma, Japan) that was set to take a picture of the tablets every minute.
RESULTS
Influence of process parameters on lag-time
The delay before the press-coated tablet releases the core was studied depending on the coating-compression pressure and layer thickness. As shown in Figure 1, a highest compression pressure involves a longer lag-time (i.e. the time needed for the shell to open and expose the core to the dissolution medium, measured as the first minute where the core is visible on the photographs). This effect was quite predictable as an increase of the compaction pressure reduces the porosity in the shell and slows down the water penetration. Another interesting effect is the high influence of the layer thickness. Indeed, the lag-time increases as the layer thickness increases. This effect might also be linked to the porosity in the band region of the tablet, that is known to be lower with a thick layer [4]. Indeed, this is the region where the water infiltrates the most during the dissolution test, particularly at the interface between the two powder fillings.
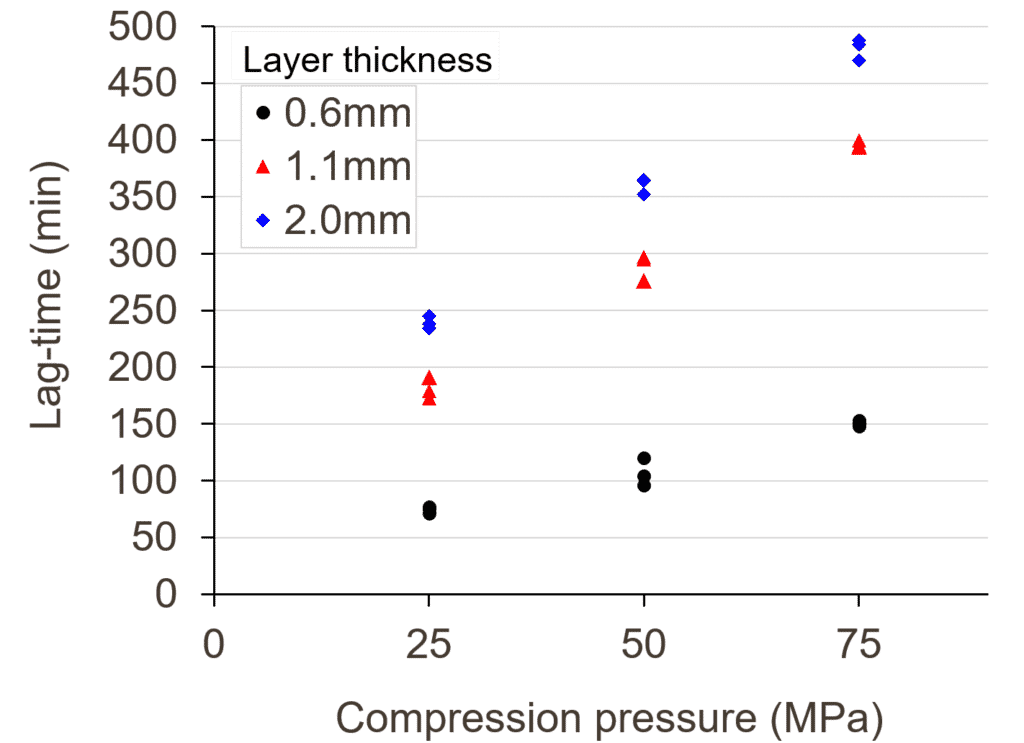
These results show that even with a given shell formula, one can reach a wide range of lag-times from 1 to 7 hours just by selecting a right pressure and layer thickness. Another result, not detailed in this abstract, shows that this range can even be wider by changing the band thickness.
Process parameters influencing the release kinetics
Apart from the lag-time, the opening mode and drug release were measured in the experiments. It was observed that the press-coated tablet can open in various modes, even with an equal lag-time. This was clearly evidenced the joint surveillance using the camera and observation of drug concentration. For instance, the shell can open by a clear detachment of a whole layer (figure 2.A), resulting in a full exposure of the core to the dissolution medium and thus a very fast burst effect and a release time inferior to 10 minutes. On the other side, instead of the whole layer, a fragment of the band can detach from the shell (figure 2.B). This creates a tight window for the active substance to dissolve and a limited exposure to the dissolution medium. This opening mode leads to a slow release, up to 60 minutes to dissolve the dose of API. The occurring opening mode is dependent on the choice of the process parameters : compression pressure, layer thickness and band thickness.
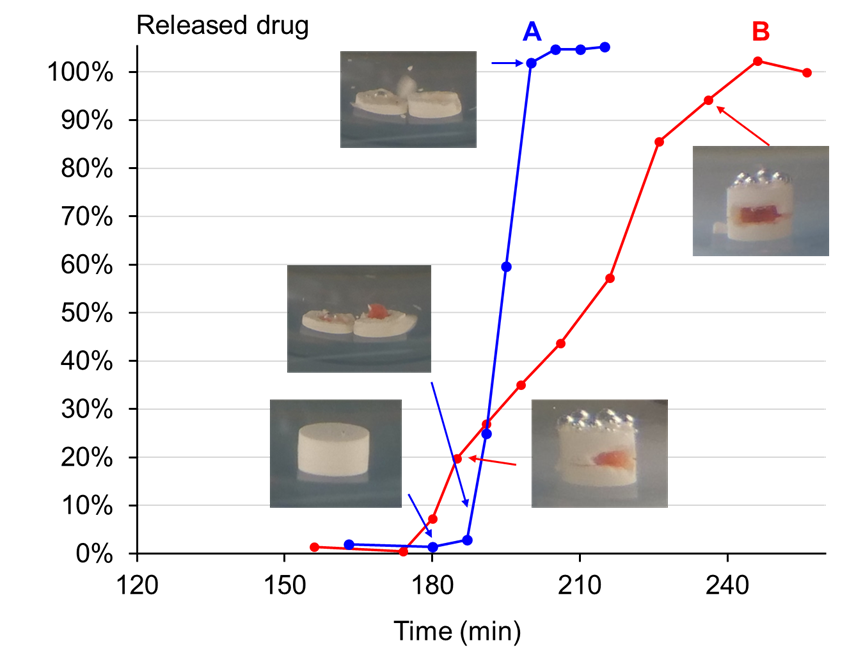
CONCLUSION
In this study, it was shown that process parameters and geometry of a press-coated tablet have a strong influence on the delay and way of opening in dissolution test. This shows the interest of visually observing the dissolution in addition to the drug release data.
A wide range of lag-time is reachable without changing neither the shell nor the core formulations, by increasing the compression pressure, layer thickness or band thickness. Parameters like pressure or layer thickness have a major importance when designing a press-coated tablet, as the release kinetic is a critical quality attribute coming with specifications to respect.
Thus, they are important levers to play on when designing this type of tablet, to reach the wanted release attributes with control.
REFERENCES
[1] E. Fukui, K. Uemura, et M. Kobayashi, Journal of Controlled Release, vol. 68, no 2, p. 215‑223, aug 2000
[2] S.-Y. Lin, M.-J. Li, et K.-H. Lin, AAPS PharmSciTech, vol. 5, no 4, p. 25‑29, dec. 2004
[3] U. Conte, L. Maggi, M. L. Torre, P. Giunchedi, et A. L. Manna, Biomaterials, vol. 14, no 13, p. 1017‑1023, oct. 1993
[4] L. Picart, V. Mazel, A. Moulin, et P. Tchoreloff, International Journal of Pharmaceutics, vol. 596, p. 120260, mar 2021
Léo Picart 1 2, Vincent Mazel 1, Aline Moulin 2, Pierre Tchoreloff 1
1 Univ. Bordeaux, CNRS, Arts et Metiers Institute of Technology, Bordeaux INP, INRAE, I2M Bordeaux, F-33400 Talence, France, vincent-mazel@u-bordeaux.fr
2 Skyepharma Production SAS, 55 rue du Montmurier 38070 Saint-Quentin-Fallavier, France



