Learn how Microfluidizer® Technology could boost the effectiveness of your drug!
The low bioavailability of active pharmaceutical ingredients (API) is one of the major hurdles in the development of oral drug formulations. Indeed, poor solubility and/or permeability of therapeutic molecules results in low preclinical efficacy that can sometimes lead to the premature abandonment of some lead candidates with high therapeutic potential. Microfluidizer® Technology can however help to improve features of active ingredients!
What is Microfluidizer® Technology?
Microfluidizer® technology is a dynamic high-pressure process wherein a liquid stream, which can be an emulsion or a suspension, passes through micro-channels towards an impingement area where the fluids flow and interact. If required, the resulting emulsion or suspension can be spray-dried to remove the liquid and produce a dried particulate that is orally administered.
Microfluidizer® technology can be used for:
- Production of nano-emulsions, liquid-liquid systems where one liquid is physically dispersed into another.
- Deagglomeration and particle size reduction of a solid in a liquid.
The Microfluidizer® Processor acts as a large pump that forces the material through a very small hole. Operating pressures start at approximately 3.4 MPa/500 psi and can reach up to 200 MPa/30,000 psi. The pressure varies according to each application in order to obtain the desired particle size distribution.
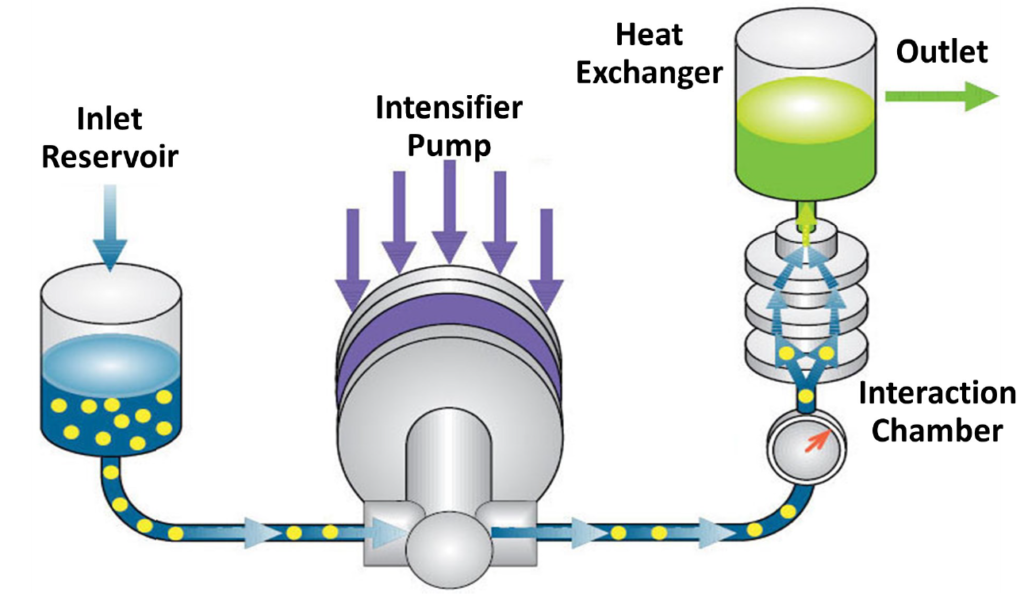
Source: Microfluidics International Corporation, Poster AAPS 2015, number W5343
The sample is first poured into the inlet reservoir. The intensifier pump has two movements: suction and compression. During suction, part of the sample is aspirated into the processor thanks to a one-way valve. The compression stroke follows and pushes this sample portion through the Interaction Chamber™. It then moves through the jacketed cooling coil (or heat exchanger according to the model), to be cooled. The sample is then collected.
The Interaction Chamber™ is the core part of the equipment. It is a continuous micro-reactor that uses turbulent mixing, localized energy dissipation, and fixed geometry to create uniform particle sizes. Within the Interaction Chamber™, the product is subjected to both high impact and shear forces that lead to particle size reduction / cell crushing. The micro-channels can measure up to 50 microns, creating micro-volumes that collectively experience a pressure profile and therefore, uniform shear application. The factor that contributes the most to particle size reduction is shear. The specific and unique design of Microfluidizer® technology Interaction Chambers makes it possible to achieve particularly high shear rates compared to other technologies.
With respect to the design of the Interaction Chamber™: the outer Chamber is made of stainless steel and the interior is either polycrystalline diamond or aluminum-oxide ceramic, compatible with pharmaceutical applications.
There are two types of Interaction Chambers:
- Y-type: generally used for liquid-liquid emulsions;
- Z-type: generally used for cell disruption and dispersion (for example reduction of particle size).
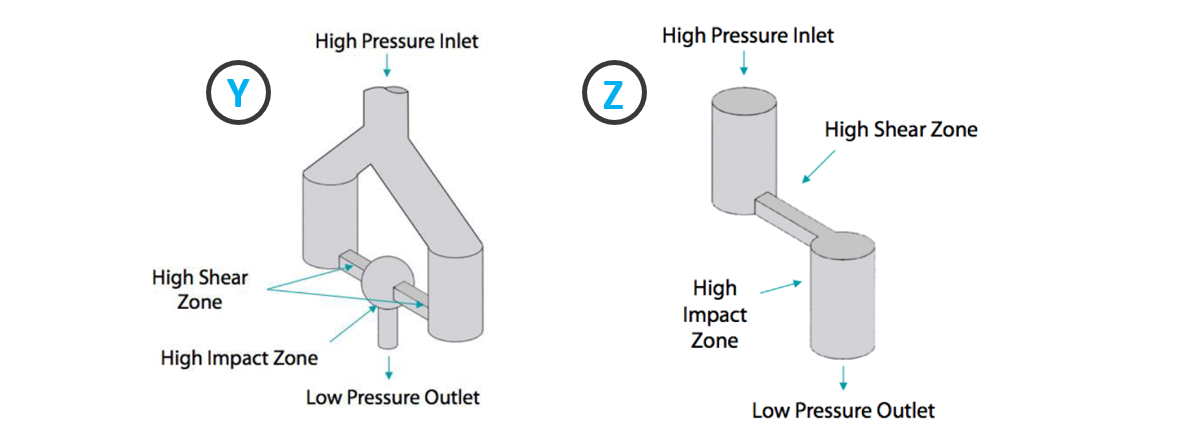
Source: Microfluidics International Corporation
Example of successful application at Skyepharma: increase of the oral bioavailability of BCS class II and IV APIs – IDD® DISSOCUBESTM technology and Triglide® drug product
Since several decades the drug discovery process is influenced by automation technologies, such as High-Throughput-Screening, combinatorial chemistry, and computer-aided drug design. The drug candidates emerging from these screening programs possess a very good efficacy due to the good fit with the target-receptor geometry. Unfortunately, many of these highly active drugs are poorly soluble. The majority of the drugs in the development pipelines of pharma companies have solubility and related oral bioavailability problems. Poor solubility of drugs is oftentimes associated with poor bioavailability, in particular after oral administration.
But already long before one of these compounds can reach the market it needs to be formulated for the preclinical studies and for the pharmacological activity tests. Therefore, it is a great challenge for the pharmaceutical development to create new drug delivery systems and formulation approaches to overcome the solubility and bioavailability problems of these drug candidates.
This challenge can be addressed with our IDD® DISSOCUBESTM technology, using Microfluidizer® process, as previously described. It allows particle size reduction, which is a universal approach to improve the bioavailability of poorly soluble drugs. The decreased particle size of drug nanocrystals leads to a distinct increase in surface area. Due to the increased surface area the rate of dissolution will be proportionally raised, leading to a better absorption of the poorly soluble drug.
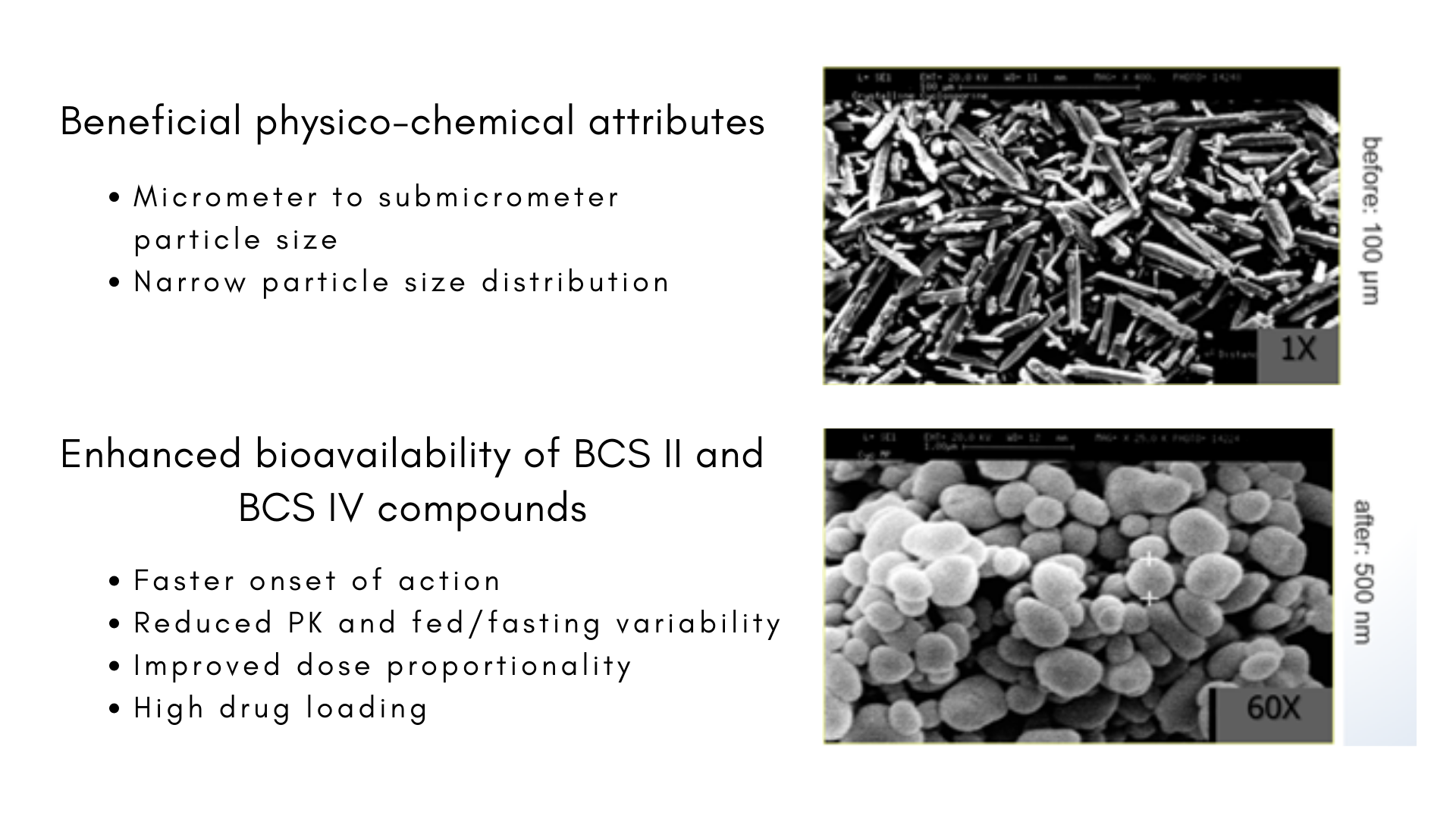
Thanks to this technology, Skyepharma developed fenofibrate drug product (Int. J Nanomedicine 2006, 1(2), 129-147), now commercialized on the US market as Triglide™.
Skyepharma is so able to develop and commercially manufacture oral dosage forms with improved bioavailabilities thanks to its Microfluidizer® Technology expertise in API particle size reduction.
Towards new applications in oral nanomedicine: establishment of a collaboration between Skyepharma and Lyon1 University.
For optimal bioavailability, APIs should have a high solubility but also a good permeability at the gastrointestinal barrier level. Oral forms are not suitable for APIs that do not have these properties and the development of conventional more complex formulations is required.
Many formulation strategies have already been developed in this direction. For example, the use of conventional colloidal vectors (micelles, liposomes, polymeric nanoparticles, etc.) may solve this type of problems. Nevertheless, these systems present aggregation, instability and preservation issues associated with uncontrolled release of APIs but also problems of toxicity of certain constituents. In addition, manufacturing processes often require the use of organic solvents and remain difficult to industrialize.
On the other hand, lipid nanovectors have a strong potential for oral administration of APIs. Due to their lipidic nature they allow efficient encapsulation of low soluble APIs in digestive fluids and the significant improvement of their passage into the blood thanks to their surface properties, thus leading to increase the concentration of API at its optimal site of action. Studies have thus been able to show the improvement in the effectiveness of APIs administered in the form of oral lipid nanoparticles for antiviral, anticancer and antibiotic or antihypertensive treatments… (J. Control. Rel. 2020, 321, 669-709; Pharmaceutics 2020, 12 DOI: 10.3390/pharmaceutics12030288).
In addition, these lipid nanovectors are biocompatible and biodegradable and therefore present very low toxicity. Finally, these lipidic nanoparticles can be produced by Microfluidizer® Technology.
Skyepharma & LAGEPP
In this context, Skyepharma is launching a collaboration with LAGEPP Laboratory (Laboratory of Automatic control, chemical and Pharmaceutical Engineering), withing Lyon1 University, led by Professor Stéphanie Briançon.
We are very proud to start this collaboration with Stéphanie Briançon’s team
At Skyepharma, we have a strong willingness to direct our R&D efforts towards promising scientific themes for the development of innovative and high-performance oral formulations, for the benefit of our customers. The implementation of this project, named “NanoMicS” is a first step forward.
Aline Moulin, Pharmaceutical Development Director
LAGEPP laboratory develops research activities in the field of chemical and health engineering with a recognized expertise in the field of galenic formulation, the design of innovative forms and encapsulation systems, associating physico-chemical aspects and process expertise. Work has been developed on the formulation of nanosystems by Microfluidizer® Technology, with laboratory equipment similar to the industrial equipment available at Skyepharma. LAGEPP also has a strong expertise in the drying processes of dispersed systems (spray-drying, freeze-drying…).

NanoMicS Team



